
We make "YOUR FUTURE"
[ Purpose ]
The effect of alternating field on enzymatic activity was examined with trypsin.
[ Materials ]
Buffer:50mM Tris-HCl,10mM CaCl2,pH8.0
Trypsin:Trypsin EDTA solution for cell culture
Enzyme substrate:100mM BAPNA ( Benzyl-DL-Arginine-p-nitro anilide ) DMSO solution
Stopping solution:33% (V/V) acetic acid solution
[ Overview of experimental method ]
200μl of trypsin solution for cell culture diluted 10-fold with a buffer solution was placed in an F8 maxisorp immunomodule (Nunc).
Then, 2μl of 100mM BAPNA solution was added, and immediately an air-core coil (Solen air-core coil(18AWG) 12.0mH) was placed in the center of the Helmholtz coil as shown in Photo 1,
and reacted at room temperature for 10 minutes under magnetic field exposure at each frequency (function generator : KUMAN).
The reaction was stopped by adding 33% acetic acid.
Thereafter, the absorbance at 405 nm was measured with an iMark immunoreader (Bio-Rad).
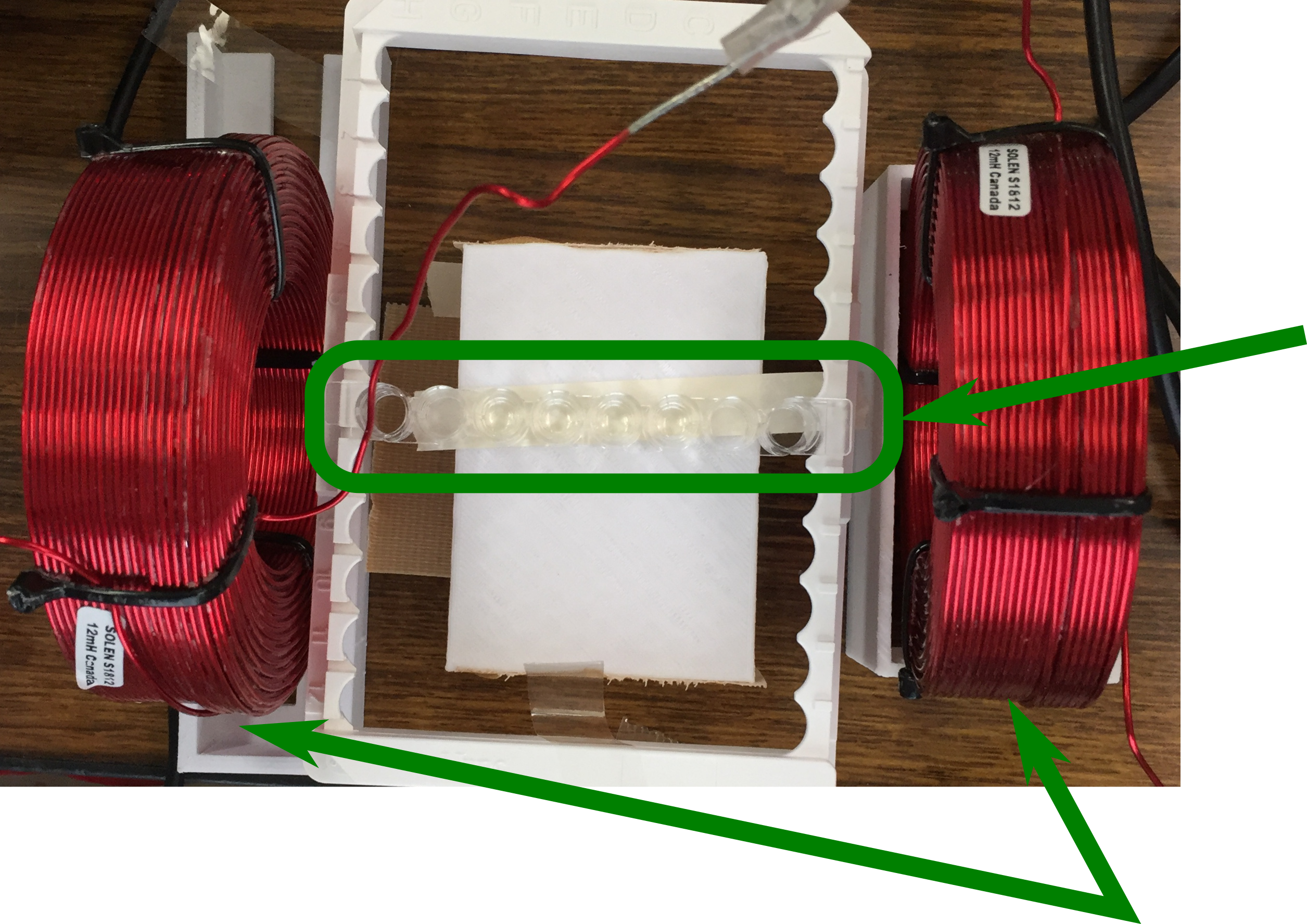
Photo 1 F8 Maxisorp Immunomodule air core coil
[ Results ]
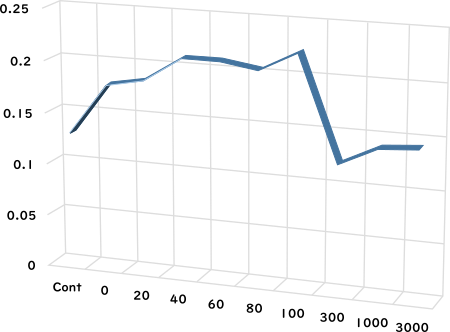 Trypsin activity (A405)
Applied AC frequency
Effect of alternating magnetic field
Trypsin activity (A405)
Applied AC frequency
Effect of alternating magnetic fieldon trypsin activity
[ Discussion ]
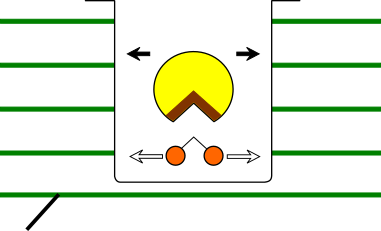

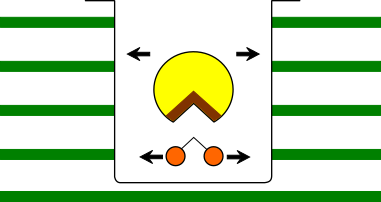
♢ The reason for increased enzymatic activity at 0-100Hz ♢ In the magnetic field, it depends on the charging state of trypsin and BAPNA in a pH8 solution, BAPNA (which has a small molecular weight) moves a long distance per unit time than trypsin. BAPNAs' moving distance may correspond to the size(large) of the active site region of trypsin. As a result, the number of contacts beween the trypsin active site and BAPNA increased, and the apparent enzymatic activity was enhanced.( Figure 1. )
♢ The reason why the enzymatic activity level was equivalent to the non-applied state above 300Hz ♢ In the case of higher frequencies, the moving distance of BAPNA per unit time getting short, and the number of contacts between the trypsin active site and BAPNA was almost the same as in the non-applied state. As a result, the enzymatic activity was almost the same. ( Figure 2. )