| ■Diagnosis Technology |
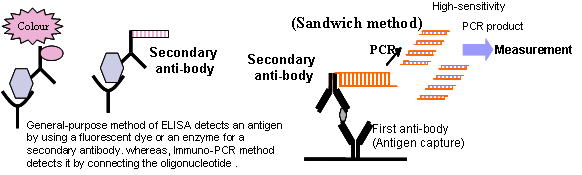
| Immuno-PCR |
 |
| ■Development process |
The “imTag PCR” applies the technology of “Immuno-PCR” to detect
an antigen of an existing infinitesimal antigen in a sample. This “Immuno-PCR”
is the technology that Sano et al. reported in 1992. It is a detection
technology that has both peculiarity of antigen-antibody reaction and the
superior detection sensitivity that PCR method has. In this way, it was
superior technology. But there were some problems in detection sensitivity
and quantification.
We have resolved these problems by performing improvement of solid-phase
materials and blocking agent, nucleic acid tag connected in an secondary
antibody and sell it as a kit. |
| ■Performance |
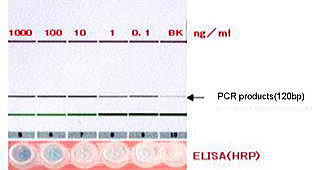
Confirmation of high sensitivity |
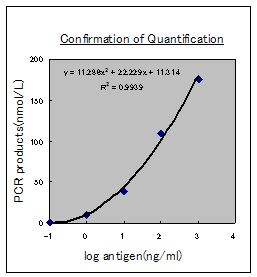 |
“imTag-PCR” enables to get more than 1,000-times sensitivity
compared with the conventional way(ELISA).

《More adaption》
・Infectious and allergic diseases
|
| ■The characteristics of “Immuno-PCR” |
|
ELISA |
Immuno-PCR |
Superiority |
| time |
1~4hours |
Within 30 min. |
Swift |
| sensitivity |
>ng/ml |
<pg/ml |
High sensitivity
(>1,000times) |
| other merit |
|
It can measure plural items
at a time |
Reasonable
accuracy |
|
| ■Product |

Brand name: “imTag PCR”
|